Why are we different?
We will always regard Cherish life Care for life Create life as the cornerstone of our business; and we regard the greatest contribution made to society as an important measure of the success of 3SBio.
3SBio Group has always been committed to the development of innovative, safe and effective drugs to treat the world’s most challenging diseases.
As medicine is a tool for treating illness and saving people, it is a special commodity. The public has devoted more attention and expectations to pharmaceutical companies. The products we provide are for human health. The history of human development is, in fact, a history of struggle between humans and diseases. We need better medicines. It is now..., because living healthily is the common pursuit and dream of us, our family and friends.
World Pharmaceutical Development
-
1980s
Life-saving drugs
Anti-infective, cardiovascular disease, metabolic drugs and hormone drugs
-
1990s
Drugs for quality of life improvement
Antilipemic agents, antipsychotic drug and ED therapeutic agents
-
21st century
Biological medicines for targeted treatment
Autoimmune diseases, tumors and other refractory diseases
Chinese biopharmaceutical industry development
Biopharmaceuticals are therapeutic proteins produced by recombinant DNA technology
• The main categories include:
– Erythropoietin (EPO)
– Growth factor
– Insulin
– Interferon
– Interleukin
– Monoclonal antibody (mAb)
Compared to chemical drugs, the molecular structures of biopharmaceuticals are larger and more complex
The historical gap between China’s chemical pharmaceuticals and the world’s pharmaceuticals is a hundred years, and the historical gap between China’s biopharmaceuticals and world pharmaceuticals is ten years.
In recent years, China's biopharmaceutical market has performed significantly better than the overall pharmaceutical market and is expected to maintain strong growth momentum in the future;
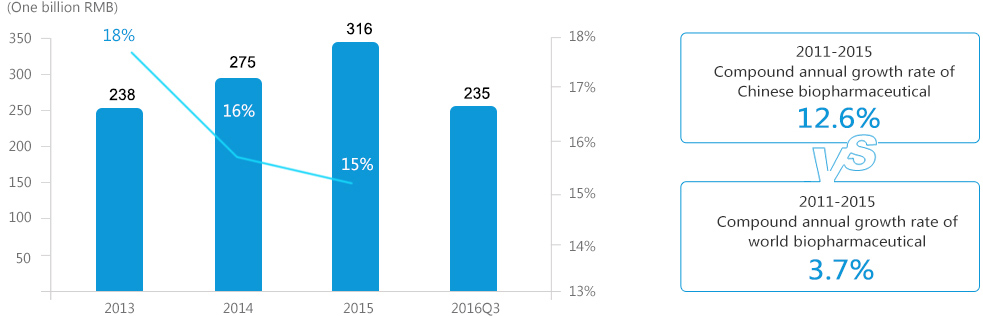
Compared to the global market, China’s biopharmaceutical market is still at an early stage of development.

Chinese government's policy support
The China Food and Drug Administration issued the first biosimilar reporting route in March 2015, raising the threshold for the approval of biosimilars. National strategic emerging industries and biopharmaceutical industry in the 13th Five-Year Plan:
• Biotechnology industry is included in key strategic industries
• Biopharmaceuticals are included in the key development areas
We are the forerunner of Chinese biopharmaceutical industry
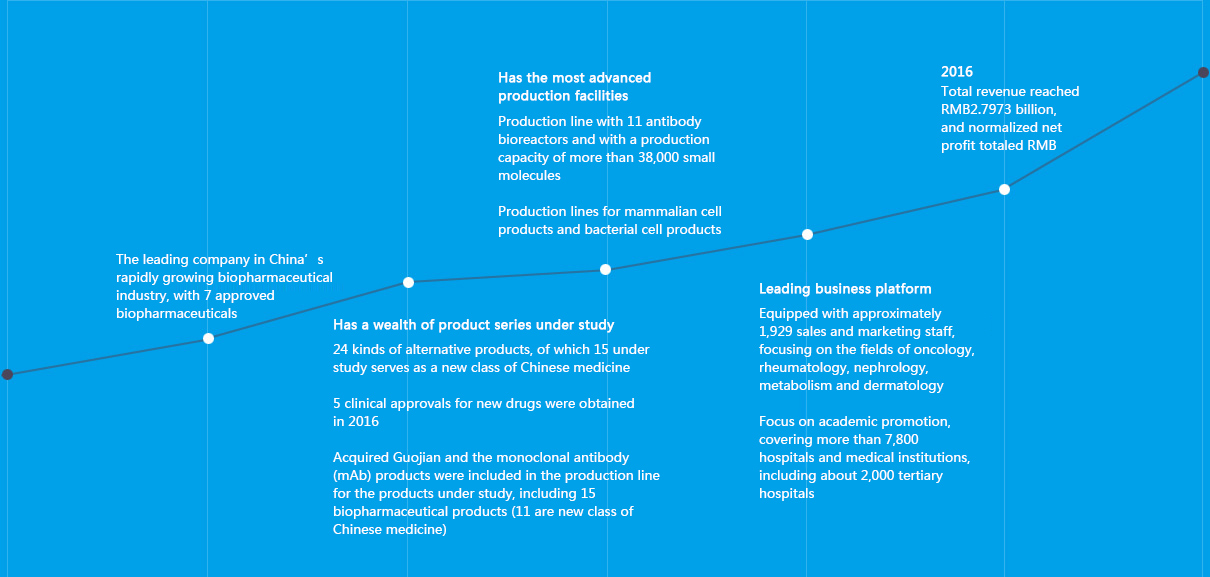
Leading core products
Etanercept
• Guojian launched Etanercept (Yisaipu) in 2005 and was the first listed product of its kind
• For the treatment of rheumatoid arthritis, psoriasis and ankylosing spondylitis
• In 2016, it dominated the Chinese market with the market share of 62.7%
• Included in Class B Drugs in 2017 National Medical Insurance Catalogue

SEPO
• Recombinant human erythropoietin product acquired in December 2014
• Improved the company’s penetration into primary and secondary hospitals

TPO
The world's only commercial recombinant human thrombopoietin (rhTPO) which is an independent research and development product
Compared with other treatments for chemotherapy-induced thrombocytopenia (CIT) and immune thrombocytopenia (ITP), it has better efficacy, faster platelet recovery and fewer side effects
Market share in 2016 was 45.6%
It is included in the drug list of emergency treatment for immune thrombocytopenia as the preferred second-line drug
Obtained market authorization from Ukraine, a member of PIC/S
Included in Class B Drugs in 2017 National Medical Insurance Catalogue
Clinical application for the new drug for pediatric indications enters the priority review channel

EPIAO
• Through calculation by revenue, it always ranks first in the recombinant human erythropoietin (rhEPO) market in China since 2002; combined market share with SEPO reaching 43.9%2 (together with SEPO) in 2016
• The only one recombinant human erythropoietin product approved by China Food and Drug Administration (CFDA) for three indications

Byetta&Bydureon
• A GLP-1 product obtained through exclusive license agreement with AstraZeneca in October 2016
• Enters the field of diabetes and enrichs the company's product line
• Innovative drug species with great market potential